
The math and physics of snowflakes
With an abundance of the white stuff outside, it behooves us to consider it in detail. After all, it’s going to be with us for awhile.
With that in mind, we asked U of M researchers Gordon Anderson in the department of physics and astronomy, and Ranganathan Padmanabhan in mathematics to help us understand what snow is―really. Anderson, formerly with Environment Canada, approaches the subject from his physics background, while Padmanabhan applies his math knowledge, each of them giving us slightly different ways to understand the nature of snowflakes.
First of all, is it true that no two snowflakes are alike?
Gordon Anderson: There is no a priori reason why two snowflakes couldn’t look alike, since all water molecules are pretty much the same―or are they? Some water molecules contain deuterium, an isotope of hydrogen, and those molecules will have a slightly different shape than ordinary water. The deuterium form is commonly known as heavy water because it weighs a slight bit more. Water in the atmosphere is a mixture of these two isotopes and they form crystals with slightly different shapes. When you mix the two together in a crystal, there will be occasional distortions in the regular spacing of the molecules that lend themselves to a new shape of snowflake. A typical snowflake will have 1,018 molecules and about 1,015 will contain deuterium, so there are an enormous number of ways in which the crystal can become distorted to form a new shape.
Ranganathan Padmanabhan: In the beginning of their initial formation―as tiny hexagonal water crystals―all snowflakes appear very much alike in shape. But as they travel through the atmosphere, there are many factors which can influence the growth of the snowflakes, like density of the water molecules, humidity, wind velocity and temperature. This is where randomness comes in. A snowflake’s arms extend outward in a random fashion as water molecules gather on it, giving birth to an infinite variety of snowflake shapes (see the third figure above). In other words, while any two snowflakes are the same at the point of their origin, they start becoming different as they grow while travelling through the atmosphere. Thus each snowflake experiences unique structural changes and gets complex patterns before it lands on Earth. To give a different example, we can say that while any two newborn babies look somewhat similar, no two grown-up adults are similar.
Why do snowflakes have six points? Why not five or eight?
GA: Water is a polar molecule―that is, it has a negative and a positive side. Two hydrogen atoms bond to the oxygen on
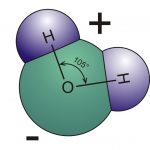
The water molecule has two hydrogen atoms and one oxygen atom, giving it a positive side and a negative side.
one side, giving that side a positive charge, leaving the oxygen side with a negative charge. Water molecules then form bonds with their neighbours as the positive side of one water molecule is attracted to the negative side of a neighbour. These neighbour-to-neighbour bonds are called hydrogen bonds. In the liquid state, the hydrogen bonds on a molecule will attract four neighbours to form a five-molecule cluster that has a tetrahedral (four-sided pyramid) shape. When water freezes, the tetrahedral clusters organize themselves into a six-sided hexagon. As other water molecules join with this crystal arrangement, the six-sided shape grows until it becomes a snowflake.
RP: Hexagonal arrangements are the most efficient ways to pack things together. Snowflakes start taking shape when six water molecules join together freezing into a crystal. There are two possible ways in which they can join. An arrangement with six molecules around each one is more efficient than a configuration with only four molecules. Nature, of course, chooses the best optimal configuration. As these tiny objects fall through the atmosphere, they gather water vapour and eventually become the familiar snowflakes. Because the water vapours come into contact with all the six sides with more or less equal probability, their arms extend outward from the hexagon still keeping the hexagonal symmetry intact. That’s why snowflakes appear to be symmetrical.
Why this particular symmetry?
RP: For artists and scientists alike, Nature is the Mother of all symmetries. In fact, symmetry happens to be a central
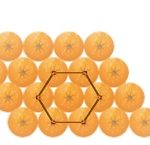
The most efficient arrangement for packing objects together is not in cubes, but more skewed or hexagonal arrays, thus reducing the spaces between to objects. This is how odd-shaped water molecules pack together in hexagonal crystals to form beautiful snowflakes.
organizing principle in Nature’s design. In particular, we can see hexagonal patterns all over: in beehives, packing spherical things like oranges, or crystals. Mathematics is the language in which these symmetries are understood and applied for human-designed projects. While an artist uses symmetry for creating an intended visual effect, a scientist tries to understand some of the secrets of Nature through the symmetry she creates. In science, one needs to observe and then predict from the observed phenomena. The undercurrent of the very process of predicting new theories from observed phenomena implies the strong belief that order can be found among the phenomena under study. This idea goes back to the Greek mathematician and philosopher Plato. The organized repetition and the visible symmetry go a long way in creating theories in physics, chemistry and biology. In fact, “group theory,” an important branch of modern mathematics, was created to communicate and to formally understand the concept of symmetry. As Galileo once mentioned, mathematics happens to be the language chosen by God to express these basic facts of science. You can replace “God” by “Nature” if you want.
Is there a difference between snowflakes formed at 0° C and those formed at -40°C?
GA: Snowflake morphology is controlled by both temperature and humidity. At temperatures near freezing, snowflakes tend to be plate-like if the air is not quite saturated, and dendritic or with many branches if the air is saturated or super-saturated. At temperatures below -3.5°C, snowflakes tend to form solid and hollow prisms. Still colder, below -10°C, they tend to form plates and dendrites again. Finally, below -22°C, prismatic, plate-like, and column forms dominate. These cold-air prism shapes are responsible for the haloes we often see in winter cirrus and other thin clouds. In truth, however, most snowflakes are glued-together mixtures of many types and it takes a bit of searching to find pure forms.






