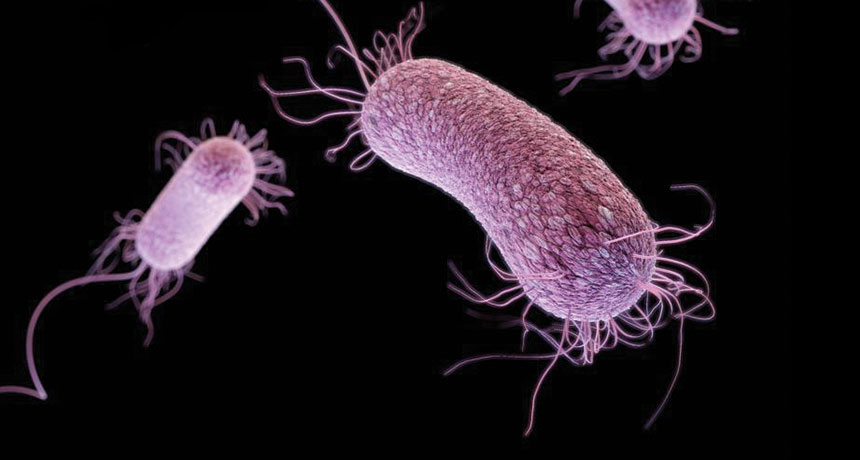
A three-dimensional computer-generated image of drug-resistant Pseudomonas aeruginosa. A new drug, PEG-2S, may be its match. // Image: James Archer, Centers for Disease Control
New weapon in fight against antibiotic resistance discovered
Scientists at St. Boniface Hospital Albrechtsen Research Centre and the University of Manitoba have developed a drug that combats 2 of the top 10 “priority pathogens” recently defined by the World Health Organization (WHO) as antibiotic-resistant bacteria requiring new interventions.
The drug, dubbed PEG-2S, has received a provisional patent, and its development is highlighted in a study published today in the Canadian Journal of Physiology and Pharmacology (CJPP). Without affecting healthy cells, the drug prevents the proliferation of a harmful bacteria that possesses a specific type of energy supply shared by a number of other bacteria.
“New drugs are not being approved because they share the same target to which the bacteria are developing resistance. We have not only defined a new and effective target, we have designed a drug to attack it without affecting normal cells,” says Grant Pierce, St. Boniface Hospital Executive Director of Research and University of Manitoba professor of physiology and pathophysiology. “The first pathogen our research team studied (Chlamydia trachomatis) has confirmed that NQR is a good target, and it is shared by many bacteria in need of a more effective antibiotic.”
The paper, “Development of a novel rationally designed antibiotic to inhibit a nontraditional bacterial target”, revealed that a variety of bacteria share a unique respiratory sodium pump (NQR) that supplies energy vital to the bacteria’s survival. The study showed that the new drug, PEG-2S, inhibits the function of the NQR pump and the production and growth of Chlamydia trachomatis bacteria. The drug is highly targeted and only impacts bacterial cells with NQR pumps and is not toxic to normal, healthy cells.
The list of NQR-possessing bacteria is growing steadily as genomic information becomes available. With more than 20 different pathogenic bacteria containing NQR, the possibility for this drug to avoid multidrug resistance through NQR inhibition represents a potential breakthrough in antibiotic design.
“The results from our collaboration are tremendously exciting,” says lead author Pavel Dibrov, professor in the U of M’s Faculty of Science. “We are currently designing PEG-2S variations and hope to tailor PEG-based antimicrobials to each specific NQR-containing pathogenic bacterium.”
Traditional targets for antibiotics are limited. No new antibiotics have been discovered since 1987 and only 2 antibiotics have received US FDA approval since 2009.
“Antibiotic and antimicrobial resistance to superbugs is a priority research direction in pharmacology. The quality and findings of this study may be instrumental in our efforts to develop new drugs and technologies that effectively address this global health alarm recently raised by the World Health Organization,” says CJPP editors Ghassan Bkaily and Pedro D’Orléans-Juste.
In regards to antibiotic resistance, Global health agencies publish a frightening number: in 35 years, if left unchecked, antibiotic-resistant bacteria will kill 10 million people annually across the globe – two million more than cancer.
“New antibiotics targeting this priority list of pathogens will help to reduce deaths due to resistant infections around the world,” said professor Evelina Tacconelli, Head of the Division of Infectious Diseases at the University of Tübingen, at the presentation of the WHO list. “Waiting any longer will cause further public health problems and dramatically impact on patient care.”
Digvir Jayas, Vice-President (Research and International) at the U of M, celebrates the collaboration that delivered the breakthrough drug PEG-2S.
“I applaud the research collaboration that resulted in this new breakthrough,” the Distinguished Professor says. “Solving the complex and evolving challenges of antibiotic resistance will put new tools in the hands of caregivers around the globe.”
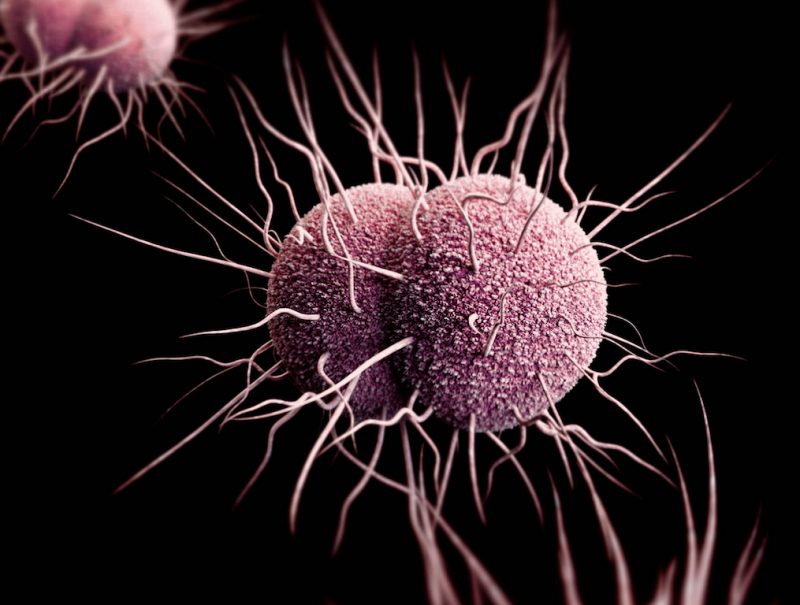
Three-dimensional computer-generated image of drug-resistant Neisseria gonorrhoeae diplococcal bacteria. // Image: James Archer, Centers for Disease Control
* WHO priority pathogens list for R&D of new antibiotics
Priority 1: CRITICAL
- Acinetobacter baumannii, carbapenem-resistant
- Pseudomonas aeruginosa, carbapenem-resistant – Targeted by PEG-2S
- Enterobacteriaceae, carbapenem-resistant, ESBL-producing
Priority 2: HIGH
- Enterococcus faecium, vancomycin-resistant
- Staphylococcus aureus, methicillin-resistant, vancomycin-intermediate and resistant
- Helicobacter pylori, clarithromycin-resistant
- Campylobacter, fluoroquinolone-resistant
- Salmonellae, fluoroquinolone-resistant
- Neisseria gonorrhoeae, cephalosporin-resistant, fluoroquinolone-resistant – Targeted by PEG-2S
Research at the University of Manitoba is partially supported by funding from the Government of Canada Research Support Fund.








Our Canadian tax dollars at work. This is excellent. I wonder about patenting such drugs, however. Should they not be freely available around the globe? Just wondering.
This drug is a major break through and should be available to all.