Superbugs meet super drugs
This story originally appeared in The Bulletin in 2008.
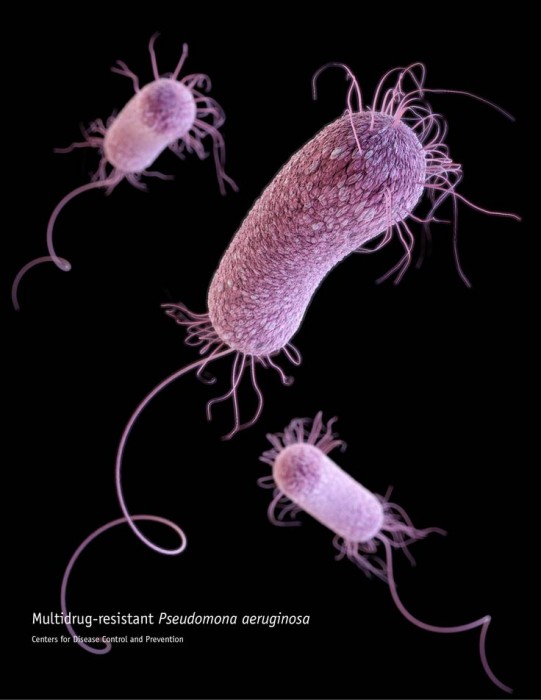
For most of its existence Pseudomonas aeruginosa has lived in soil, gangland for microbes, where it developed such a thorough array of self- defenses it may just be the most attack- resistant organism on the planet.
In the 1970s it was found in some hospitals, but today it’s reckoned to reside in every one. It’s a superbug resistant to antibiotics, and for the time being it only troubles easily-infected hospital patients.
For years it’s been a favourite subject for Medical Microbiology’s George Zhanel, and since 2006 he has collaborated with Chemistry’s Frank Schweizer in hopes of finding novel drug therapies to curtail its – and other superbugs’ – zest. They recently published a paper in the Journal of Medicinal Chemistry.
To breezily understand what they do, think of Batman. Essentially, he is just a do-gooder reliant on his tool belt; the more tools he has the better he fares in a fight. Antibiotics are the same. But Hollywood has upgraded Batman’s tools since the days of Adam West. Chemistry, however, has not been as successful at doing this until, thankfully, now.
“In the last 50 years, only three new classes of antibacterial drugs have been developed,” Schweizer said. “This does not give you many avenues to explore. So now it seems the only way to come up with a novel agent is to go back to what’s known, to some old drugs, and tweak some of them to see if you can restore their effectiveness.”
Schweizer is an expert in medicinal chemistry and Zhanel is an expert in superbugs. One day Schweizer called Zhanel and they got to talking about antimicrobials. They discussed what sort of important things need to occur in the field and eventually they talked about aminoglycosides, a class of sugar- based antibiotic that has been around since 1944.
“They are thought of as being the most rapid killers of bacteria in the world,” Zhanel said. “But they have two problems. One is that resistance to them has developed in Canada and every other country. And two, they put people into kidney failure because they are so toxic. Other than that, they are fantastic.”
The researchers developed a program to circumvent these problems. Schweizer reengineers the drug’s chemistry, and Zhanel sees if they can kill the nastiest superbugs Canadian Intensive Care Units send him without harming human red blood cells – the canary of drug toxicity
“We’re still far from bringing drugs to patients but we’ve done some tough things so far,” Zhanel said.
Zhanel’s in vitro tests of Schweizer’s drugs have shown reengineered aminoglycosides as a promising weapon. But altering their design is difficult.
Imagine aminoglycoside as an airport terminal. Each gate protruding from the terminal is a hydroxyl (a hydrogen atom bonded to an oxygen atom), and since these are responsible for the drug’s characteristic chemical reactions, any alterations bring big changes.
It used to take about 15-synthesizing steps to isolate and change a hydroxyl, which is too lengthy and costly for drug companies to care for, but Schweizer has patented a way to do it in just two to four steps.
But this chemistry can also apply to peptides. He can take a peptide 50 amino acids long, find the critical bit, and create a peptide just five amino acids long. He can now also synthesize unnatural ones that bacteria have never seen before.
In one method called “Carbohydrate- templated amino acid synthesis”, he takes a novel peptide and inserts it into a sugar scaffold that he puts on one of the terminal’s gates.
The new structure allows the drug to act as a detergent – disintegrating the bacterium’s membrane so that’s its insides spill out. It’s like blasting a shotgun at the cell wall. It’s difficult, although not impossible, for bacteria to develop resistance to this. What is more, human red blood cells have different membrane structures so the shotgun does not take aim at them.
Schweizer is also looking at aminoglycoside peptide conjugates. This method inhibits the bacterium’s main defense: enzymes that latch onto drugs and chemically neuter them.
He attaches ultrashort antimicrobial peptides to aminoglycosides, and these peptides prevent the enzymes from working, allowing the drug to attach itself to the bacterium’s RNA where it then halts its protein synthesis.
So, how do these retrofitted drugs fare in tests?
Resistance is catalogued by “minimal inhibitor concentrations” or MIC. The best drugs have an MIC of less than 1.
Neomycin, for instance, is an aminoglycoside superbugs have developed resistance to. When faced with the superbug like MRSA, Neomycin had an MIC of 512. It is, in a word, useless. But after Schweizer tweaked it, the MIC dropped to 8. With P. aeruginosa, the MIC went from 512, to 32. Schweizer has since gotten these numbers lower too.
“So we’ve restored their activity, we think they’re less toxic, and now we need to do some mechanism studies, do some animal efficacy studies, some more toxicity studies, and then see where we are after that,” Zhanel said. “So yeah, this is some cool stuff, but now we want to take this to the next level.”
Research at the University of Manitoba is partially supported by funding from the Government of Canada Research Support Fund.