Outsmarting Injury
Long before Kristine Cowley [PhD/98] received a prestigious Canada Research Chair at UM, she was a wheelchair track athlete at the 1992 Paralympics in Barcelona.
“My competitors and I were struggling with overheating,” says Cowley (then known as Kristine Harder), who had sustained a spinal cord injury in 1987 at the age of 20.
“Our autonomic nervous systems couldn’t transmit the signals to increase our heart rates and make us sweat. I started to wonder if there was some way to activate neural pathways in the absence of transmission from the brain.”
Today, the Winnipeg-born neurophysiologist – who set two world records at those Paralympics – is a leading researcher on how to increase exercise capacity in people living with spinal cord injury.
In 2021, she received five years of funding from the Canadian Institutes of Health Research as a Canada Research Chair in function and health after spinal cord injury.
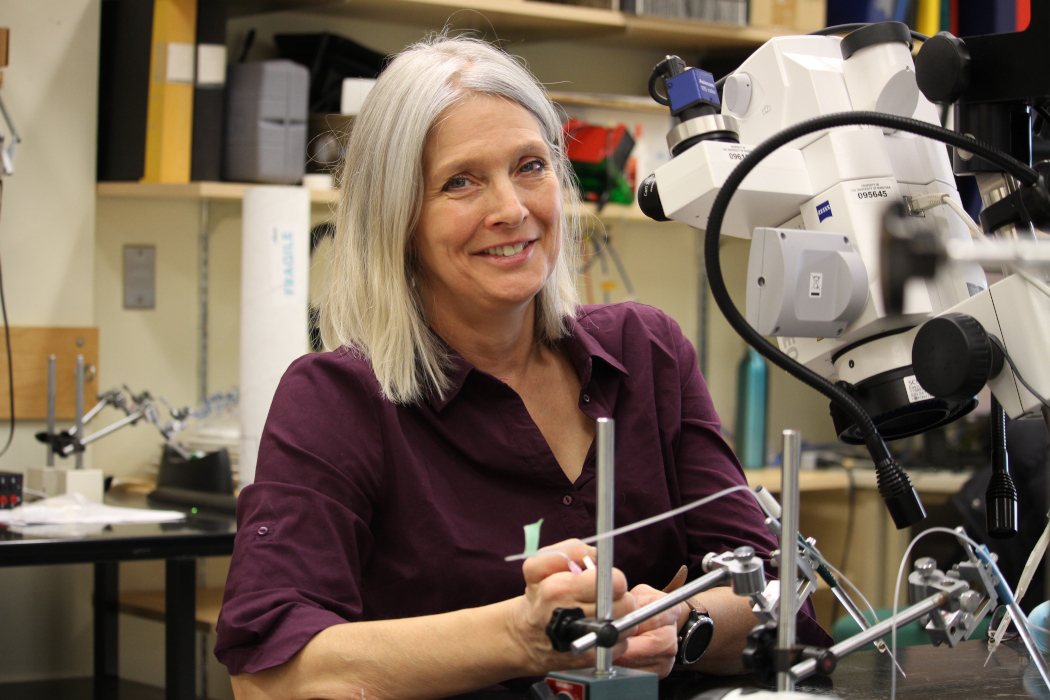
“When you exercise, activating something like the heart normally involves co-ordination between your brain, areas in your brain stem and the spinal cord. But in spinal cord injury, that communication is lost,” says Cowley, associate professor of physiology and pathophysiology and director of the Spinal Cord Research Centre at UM.
Cowley has developed a new conceptual framework, published in 2018 in the journal Applied Physiology, Nutrition, and Metabolism, to explain the integration between spinal locomotor-related neurons that produce movement, like walking, and spinal autonomic-related neurons that control functions needed to support exercise, like sweating and increasing heart rate and blood pressure.
In her lab, she uses both human and animal models to study how this type of information is transmitted. She aims to learn more about artificially activating neurons that remain functional below a person’s injury.
“There’s quite a bit of promise that we could trick the neurons below the level of injury into becoming rhythmically active. Even if people aren’t going to walk, necessarily, they might be able to perform rhythmic movements, which is a form of exercise.”
One promising approach, Cowley says, is to activate neurons through neural electrical stimulation, which has already been used to induce rudimentary standing and stepping movements in people with long-standing spinal cord injury.
“There have been reports, which are mainly anecdotal, that spinal electrical stimulation to induce stepping also improves some autonomic functions, but the pathways and neurotransmitters involved are completely unknown,” she says.
People with spinal cord injury are at high risk for secondary complications, including obesity, bone fractures, Type 2 diabetes and cardiovascular disease.
Cowley’s research with people like herself who have tetraplegia (paralysis of all four limbs, also known as quadriplegia) has looked at questions such as how many calories they expend while exercising.
Within the next 10 years, Cowley predicts, research will allow people with this form of disability to exercise for longer periods at higher intensity. That will support better overall health.
“If you could have the body function in a way that reduces the risk of sedentary-related disease, that would be a win,” she says.
“The most satisfying thing about this research is attempting to find answers to currently unsolved research problems that may help people in the long run.”