Nothing remarkable happened to Chris Carswell on that October afternoon in 2007, until he died. He was in Grade 3 at Satilla Marsh Elementary School in Brunswick, Georgia, eating spaghetti in the cafeteria when he suddenly felt tired. He put his head on the table and told his best friend to wake him when lunch ended. Then he had a stroke, then a series of seizures, and then he went into respiratory and cardiac arrest before being revived by paramedics. Seven hours later he woke from a coma in the hospital.
The stroke left a pernicious patch of scar tissue deep in his brain for the next seven years, three months and twenty-four days. This neurological scab triggered two to three seizures a day. When he tasted stale mint gum in his mouth he knew one was imminent.
Years passed. Experts came and went. Drugs were tried and failed. Every day Carswell worried one of the seizures would be the kind that never ended. Every night his mother Janet, a nurse, would wake three or four times to check on him, fearful always.
In 2014, medical tests at a Florida hospital confirmed a dire diagnosis: they couldn’t remove the tissue—traditional surgery would cause too much collateral brain damage. The next year, however, there was hope. A new surgical tool—the NeuroBlate probe—developed by University of Manitoba alumni, had been acquired by the hospital. It could do what traditional tools could not: It promised to rid Carswell of the tissue.
“It was the best news I had heard since he was nine years old, before all this began,” Janet says. On Feb. 4, 2015, surgeons drilled a pencil-sized hole into the back of Carswell’s head, slid the NeuroBlate probe deep into his brain and shot a laser precisely at the scar tissue. Before the operation Carswell had suffered more than 5,000 seizures; since the operation, not one. “The seizures changed what I could do,” says Carswell, now 17. “They took away opportunities to do certain things. Now that I’m seizure-free, I’m not afraid anymore. I’m confident again. It’s just an amazing feeling.”
THE MAGIC FROM THE NAPKIN
For decades surgeons have biopsied brains using minimally invasive techniques. In the late 1980s, Mark Torchia [MSc/97, PhD/01], a clinical scientist at St. Boniface General Hospital, was invited to watch such a procedure by fellow alumnus Dr. Michael West [BSc(Med)/73, MD/73, PhD/80], the former head of the U of M’s department of neurosurgery who established the Gamma Knife procedure—a knife-less surgery that blasts tumours with radiation—in Canada.
After the operation they went to the cafeteria and West mused that the biopsy probe should do something more—if they’re already piercing the tumour, why can’t they do anything to attack it? Torchia and West sketched something on a napkin and then parted ways.
“It sat in my head for a year or two and I thought, ‘Okay, maybe this is actually worth doing something with because I was aware of the continuous flow of patients coming in with these glioblastomas—horrible tumours. It’s essentially a death sentence,” Torchia says. “And this just started wearing on me.”
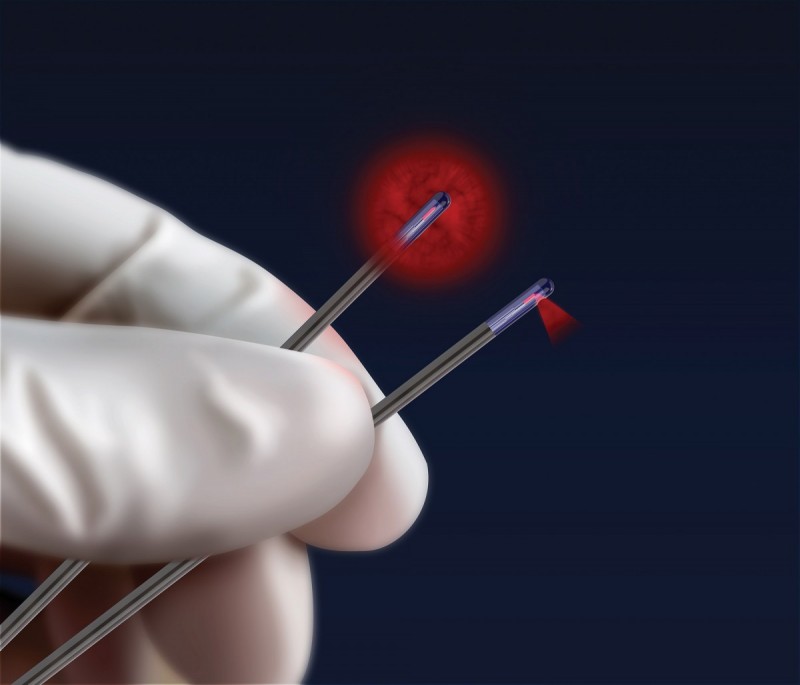
Torchia realized the probe must focus its beam at a 90 degree angle, rather than emit from all directions.
He grew to despise four words: Your tumour is inoperable. Doctors somberly say this every year to 15,000 North Americans. About 15 months later, the patients die.
Right now 100,000 people in North America have malignant brain tumours, and every year 20,000 more learn they do. Add the number of seizure patients like Carswell, and you have a wretched sum of suffering caused by inadequate tools.
The numbers tormented Torchia. He wanted to create a device that a surgeon could guide with unprecedented precision to a brain tumour, and then, using real-time information from an MRI (magnetic resonance imaging) machine, precisely “poach” the tumour with controlled doses of lethal heat produced by a laser.
The idea was too visionary.
In 1991 Torchia submitted a grant application he co-wrote with West to develop his idea. One of the world’s most renowned neurosurgeons sat on the review panel. Torchia recalls the surgeon telling him, “That’s the most foolish, simplistic and obviously-could-never-succeed device I have ever seen in my life.”
Torchia then presented to a leading MRI research team in Europe who told him his idea was impossible.
Thinking he just wasn’t explaining the idea well, Torchia sketched a prototype and showed it to the inventor of the fibre optic endoscope. But he too laughed. Literally.
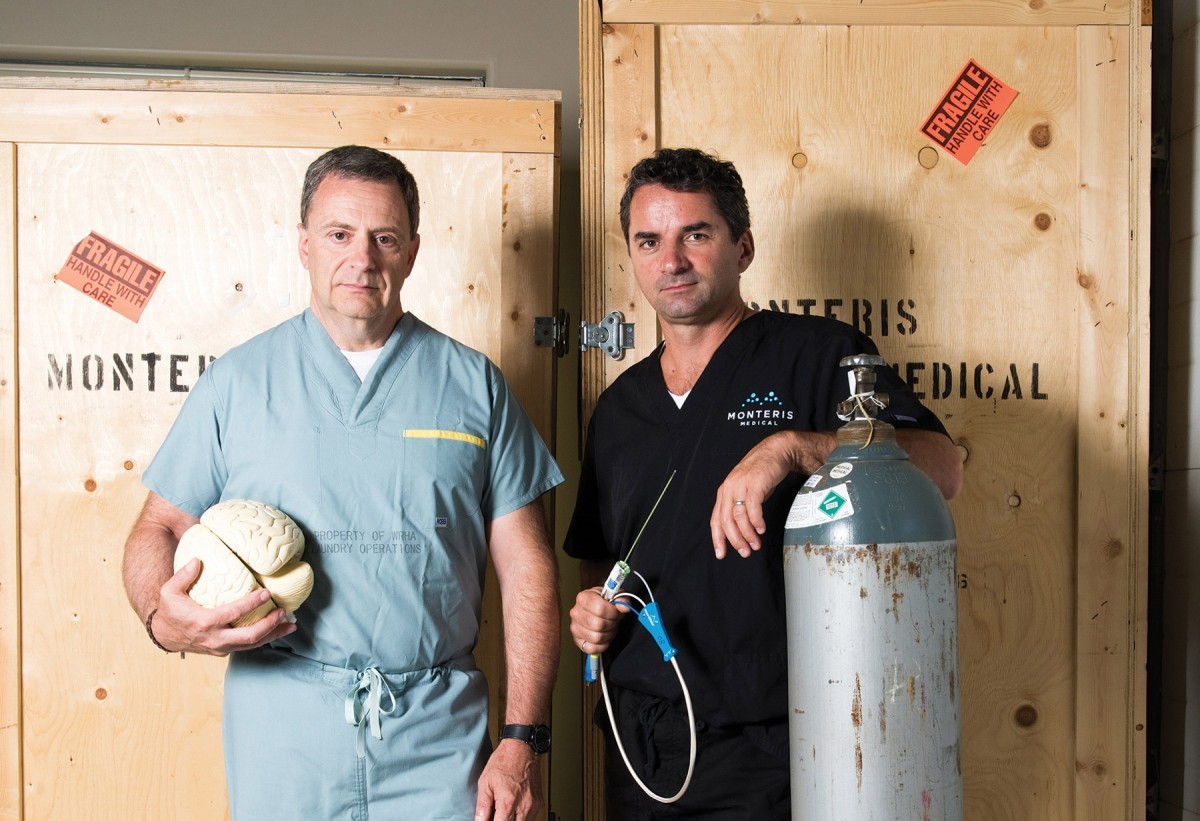
“Then I made one of the best decisions of my life,” Torchia says. He hired Richard Tyc [BSc(ME)/89, MSc/94]. In 1999 they founded Monteris Medical, a Winnipeg-based company dedicated to creating ground-breaking neurosurgical technologies.
While working at St-Boniface Hospital Albrechtsen Research Centre, they created the Monteris NeuroBlate® System, which won the prestigious 2015 Ernest C. Manning Principal Award in October 2015, and more recently won the inaugural Governor General’s Innovation Award.
INCREDIBLY TERRIFYING
Their partnership began with Torchia showing Tyc his bench prototype— a stainless steel tube that ended in a glass prism. It had a gas canister attached to cool it, and near the tip, a cassette that would run cloth across the prism to clean it. It was inelegant but on the right path. “When I saw that, well, you don’t have to be an engineer to realize it’s big. I mean, the thing was the size of your finger. It was a pretty big tube that you were going to poke into somebody’s brain,” Tyc says. “So I knew we had to be smaller. And also, that wasn’t going to work in the MRI. It was cool to look at though.”
Over the next few years the team expanded to include many other U of M engineering alumni, and the probe was slimmed to its current diameter of 2.2 millimeters, and uses materials that work in an MRI’s strong magnetic field, without corrupting its images—a crucial feature.
“I’m going to go on a limb and say we invented the first truly MRI-compatible robot,” Tyc says. “The surgeon can move the probe in an MRI while it’s imaging. So while he’s firing the laser he’s watching the treatment screen in real-time. That is pretty revolutionary.”
Torchia realized early on the laser should fire at a 90-degree angle in a narrow beam. With the NeuroBlate, the surgeon can direct lethal doses of heat to the tumour’s exact boundary, firing longer in some sections, shorter in others, killing just the cancer cells.
“This is kind of the magic of the thing,” Torchia says. “It was a paradigm shift.” By 2003, Torchia and Tyc had their first prototype. In 2008, the system was tested for the first time on a human at the Cleveland Clinic. It was one of the 10 patient procedures permitted in which NeuroBlate had to succeed in order to receive FDA approval. Torchia and Tyc were stressed.
They were confident in their design, but NeuroBlate software has the same amount of software coding as the Mars Curiosity Rover— five million lines.
“The night before, I didn’t sleep at all,” Tyc says. “I just kept thinking of everything that could go wrong. What can crash? Have we thought of everything? You know, this is brain surgery. The surgeon is trusting our technology. He’s been a part of the development process but he doesn’t understand anything about the underlying system. He’s trusting us.”
They succeeded. The FDA approved NeuroBlate for coagulation of tissue, a broad category that is now allowing the device to be used on other ailments—like seizure-inducing neurological scar tissue, or perhaps one day on breast tumours, or in the liver or pancreas.
IMAGINING TOMORROW
“There are very few visionaries in the world who can look 20 years down the road. Those who forget about what is today but imagine what could be tomorrow…obviously Mark is a visionary,” says U of M Medicine alumnus Dr. Edward Lyons, this year’s recipient of the Distinguished Alumni Award for Lifetime Achievement.
Though Lyons knows Drs. Torchia and West, he learned about NeuroBlate only this year, but this doesn’t surprise him. “A lot of successes people have, even in the medical community, go unnoticed and unannounced,” he says. “There are a lot of people who do intense, dedicated work at places like the University of Manitoba, and it doesn’t always end up being a NeuroBlate. I mean, how would you even begin to find out about the success that Manitoba graduates have had in developing new drugs, new products, new procedures?”
Indeed, many don’t know U of M engineer John A. Hopps [BSc(EE)/41, DSC/76] made crucial design contributions to the first pacemakers in the 1940s, or that today U of M researchers are poised to make seismic shifts in dementia research (see sidebar).
Lyons himself should be a household name: he and his team were the first to find clinical applications for pelvic ultrasound and quickly became a global authority on the new technology early on, driving its advancement and adoption in hospitals around the world. He’s one of the reasons we can spy on an early fetus in a womb from five weeks onwards. “Everything is possible; it’s just a matter of timing and a matter of working through the challenges. Something that was thought to be impossible in the 90s is obviously, today, feasible,” Lyons says. “You have to have an open mind in science.”
FLOATING OFF THE GROUND
Monumental impacts are rare events. It’s one thing to discover something, but it’s another to have your discovery remove “inoperable” from a lexicon.
“I wouldn’t call NeuroBlate revolutionary but it certainly is an original idea,” West says. “It impacts a very distinct group of people and it certainly has changed their lives because they had no other choices. So they would describe it as revolutionary.”
Torchia doggedly pursued this for decades because he wanted to help the helpless patients. But as grand as their vision was, he and Tyc didn’t imagine applications beyond tumours. They didn’t think they’d impact people like Carswell.
“That’s really the gratifying part,” Torchia says. “It’s a bit strange. It’s hard to describe. It’s kind of heartwarming in one sense, and in another sense it’s extraordinarily humbling. It’s like, ‘Wow, did we actually do that?’
“[Tyc] was at a surgery not that long ago in Vancouver and the surgeon there does the procedure with the patient awake. And [Tyc] said to me, ‘I was in the room, they finished, they took all the stuff off the patient and the patient had to go to the bathroom. So the patient got up and went to the bathroom.’ I was like, crap! You know, there was basically no recovery time. He just hopped off the bed. And you’ve changed the whole paradigm of something.
“In some ways, it feels like a lot of weight on you. But the more that it’s accepted and the more that it is used successfully, that weight changes to floating off the ground.”






What a wonderful story. We are world-class!
Sean Moore, you told the story in an extraordinary way. Thanks for that.