Happy birthday penicillin
Penicillin turned 85 this year. Discovered by Alexander Fleming, he published his findings in the British Journal of Experimental Pathology, and on the paper’s fiftieth anniversary, in 1979, the journal revisited his work and wrote a dedication to it.
“Thus, this journal,” Lawrence P. Garrod wrote, “takes pride in reprinting after 50 years the paper which has such momentous eventual consequences, not only a revolution in therapeutics, but the establishment of a new and very profitable industry and undreamed-of advances in microbiological research in new directions. We salute the memory of its author, which despite lavish adulation remained outwardly a modest and unassuming man.”
Where has this therapeutic revolution taken us today? Is it time to let penicillin retire? What other drugs can take its place, and what do we do about antibiotic resistance and the superbugs that sprung from our misuse of these antibacterial drugs?
UM Today sought the wisdom of two leading researchers in the field of antibiotic drug discovery and antibiotic resistance: Dr. George Zhanel and Dr. Frank Schweizer, who have been collaborating with each other since 2006.
Zhanel is a professor in the department of medical microbiology at the University of Manitoba, and the director of the Canadian Antimicrobial Resistance Alliance (CARA), a national microbiology research group dedicated to studying infectious diseases, antibiotic usage, and antimicrobial resistant pathogens.
Schweizer is professor in the department of chemistry, and an assistant professor of medical microbiology at the University of Manitoba. In 2012, the Manitoba Health Research Council awarded him a five-year Manitoba Research Chair. He holds ten patents.
Is it time to retire penicillin?
GZ: No. Penicillin is – the way we understand it – an old antibiotic. But it is safe and for very specific uses, such as syphilis, dental infections and strep throat, it’s still a good drug. The problem is, 10, 20 years ago we used to use it for dozens of infections. Today it’s down to a few select infections. So we don’t want to retire it but we need new, fancier, versions of penicillin because of all the resistance that is occurring to the penicillin we have.
FS: Penicillin is still used but nowadays we have novel analogues of penicillin. We basically adjust penicillin to what the bugs are doing. So it still has its value. The warhead of penicillin is linked a unique beta-lactam structure. However, over time bacteria have learned how to inactivate the warhead – they cut it into pieces (to watch a technical video showing this, click here). By doing that, the drug is no longer active. What you can do, and what some drug companies now do, is you can add an inhibitor to penicillin that prevent the bacteria from cleaving the beta-lactam structure.
Why is it so difficult to develop new antibiotics?
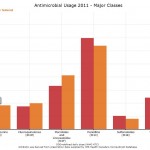
Canadian Antimicrobial Resistance Alliance chart showing Manitoba’s antibiotic use compared to national trend
FS: You know, to be honest, antibacterial drug development has been very successful in the early stages. Life expectancy is high nowadays. Go back 100 years and we have a life expectancy of 40 or 50 years. So the development of antibiotics is one of the main contributors that has resulted in a dramatic increase in our life expectancy. However, many pharmaceutical companies are no longer really interested in developing new antibiotics and the reason for that is because it’s less profitable. If you compare antibiotics to another drug, like an anti-cancer drug, you will typically take that drug for years as a patient. But for antibiotics, typically, the patient takes it for a week perhaps, and that’s probably just once in your life you get that infection. So the return for drug companies is much lower and of course there is the huge costs for developing a drug. Some people say 1.7 billion dollars to develop a drug – from drug discovery, animal studies to human trials. On the way somewhere you patent your drug, which gives you 20 years of protection, but very often after you finish the clinical trials you have thirteen or fourteen years of development behind you and so you have only seven or six years to earn money on this product.
GZ: Because the bacteria are smarter than we are. Very simply, the bacteria have been around on the planet for millions and millions of years. They divide and make new babies every 20 minutes, and it is survival of the fittest: if they are exposed to some sort of toxic insult like an antibiotic, they adapt. The susceptible ones die off. The ones that have developed resistance live and make resistant babies. And because they change every 20, 30 minutes they adapt virtually faster than any other living thing on the planet. So we can put our best minds together to try and stay one step ahead of them, but we’re losing because they can adapt very quickly. I’m also convinced they have a bit of a brain and they are doing a bit of thinking – they know what we’re doing. They know what we’re doing in terms of vaccines. They know what we are doing in terms of antibiotics. And they are working very hard to resist and develop resistance to what we are doing. So we are playing catch-up, and our goal is to continue playing catch-up with a variety of strategies, and one of the most important strategies is to develop new antibiotics to kill these new, resistant organisms.
It sounds like you’re in a bit of a chess match against these bacteria. How does that feel?
GZ: It’s exhilarating because you can’t wait to go to work the next morning to find what the day will bring, because you go to work and all of a sudden a colleague emails you saying there is a new diagnostic test that identifies these new superbug pathogens very, very quickly. You come to work and you realize that a new multi-drug-resistant organism is killing patients somewhere in the world and that organism is coming to Canada and we have to get ready. It’s exhilarating because the work you’re doing designing new antibiotics may come to fruition. But it’s frustrating because at times you think you took one step forward with a new vaccine or antibiotic and before you know it the organism has developed resistance and you’re back even further than when you started. And it’s also humbling, realizing that we have some of the greatest minds in the world – not just at the University of Manitoba but across Canada and other parts of the world – and we’re trying to solve this problem and it’s difficult. As we solve a problem, two more problems surface.
FS: Maybe a microbiologist enjoys it more. From a chemistry perspective, yes, of course it’s a permanent challenge because bacteria have been around for a long time. Recently some researchers isolated some bacteria in a cave where no human has ever been. The cave was sealed. And they found bacteria in there that have never been exposed to our modern antibiotics but already knew how to overcome the antibiotic action. So they are far ahead of us. As a chemist, in a way, it keeps us in business. Resistance will always occur to any combination you make in the lab. They only thing you can do is to develop agents that will give you couple of years of protection before they select for resistance.
Last time I checked in you were working on aminoglycosides and cooking up new recipes. Back then, aminoglycosides were a great antibiotic but were very toxic. Are there any developments on this front or have you abandoned them to investigate new drugs?
GZ: We have done both. We have continued working on our aminoglycoside analogues and we are committed to that and the reason is we think this is the most powerful drug class on the planet in terms of killing bacteria. But resistance has occurred so we are trying to make changes in the molecule to give us activity against these resistance organism and we are making headway. We have a couple of molecules that are significantly more active than the older drugs but we continue to fine tune to get even more and more and more activity.
At the same time we have also ventured other types of antibiotics. We are working on things like cationic peptides, lipids, trying hybrids, which is hooking two types of antibiotics together. So we’re working on a variety of approaches because we realized we can’t put all of our money into aminoglycosides. We have to put some energy into other therapeutic classes. Dr. Schweizer recently brought in a post-doc from Singapore and his expertise is nanotechnology in gold. So we have been trying to fuse antibiotics onto gold nanoparticles, and assess their activity because the thinking was, can we get these gold particles into the antibiotic-resistant bacteria and then liberate a Trojan horse antibiotic.
The hypothesis was that gold would be able to rapidly penetrate the bacterial cell because it has favorable properties. Bacteria cells frequently allow various high and low mass metals in – they need things, magnesium, calcium, etcetera. So the thought was the gold would rapidly penetrate the cell because the bacteria would see it as something good, and then the Trojan horse would be set free. So we’re trying to sneak up on these organisms and to out-think them and try novel things and see how they respond.
Dr. Schweizer, can you tell us more about this from your chemistry perspective?
FS: There is a current trend in drug discovery to present drug molecules on nano surfaces. Gold nanoparticles have been shown to have suitable properties as drug delivery systems but so far very little data exist whether this approach will work for antibacterials.
Why gold?
FS: You could use many other nano surfaces but we decided to use gold surfaces because a lot of chemistry has already been developed on gold and since we are not nanoparticle experts we, use a concept others have already developed in a different context and now try to apply it to antibiotics. Gold is ideal for attaching certain antibacterial compounds so the chemistry is known. But of course you could use silver nanoparticles or other types of nanoparticles as well.
It sounds like you are trying to do a paradigm shift in your thinking. Would you agree?
FS: Um, yes. This is my own experience: we have introduced over the last four or five years a variety of subtle modifications on aminoglycoside antibiotics and other antibacterials. Yes, we get some interesting results and in some cases were able to restore activity in some of these agents but I don’t think this approach will provide us with enough time before resistance reoccurs. We are currently working on antibacterials that have multiple, more than three modes of actions in one molecule. Our hypothesis is that the likelihood that bacteria will develop resistance to these kinds of agents is reduced. It’s not impossible.
There’s nothing impossible for bacteria – if I have learned anything so far. But we could at least gain more time.
Of course, obviously if you really want to cope with this problem you have to limit the use of antibiotics drastically. In veterinary applications and the farming industry they are overusing these compounds and it’s no wonder we have resistance development at an alarming rate. So I think a combined approach has to be selected you restrict the use of antibiotics and you come up with new ways of combining multiple modes of actions in a single antibiotic. However, antibiotics displaying multiple modes of action may have their own problems associated with their non-selective character which may result in undesired side effects. Coming back to penicillin, the nice thing about penicillin is that it has a single mode of action – it inhibits the enzyme-catalyzed cell wall synthesis in bacteria, a process which is unique to bacteria and doesn’t exist in humans so you don’t expect any side effects.
Last time we spoke, a bug of particular interest to you was Pseudomonas aeruginosa. What’s developed on that front?
GZ: It’s made huge headway. Pseudomonas aeruginosa — currently in hospitals across Canada, the United States, and the western world, like the EU and countries like Japan – is one of the top five pathogens causing hospital-acquired pneumonia, hospital-acquired infections of the urinary tract and wounds. So it is a major, major pathogen you acquire in hospitals. It lives in the hospitals, it lives in the water. Loves to infect patients wherever there is water, whether it’s in the eye, whether it’s in a wound that has water or the urinary tract that has water, or the lungs, that have water. It is a huge problem. It is a major, major pathogen. A lot of patients suffer tremendously and die when they develop these Pseudomonal infections.
The big problem with Pseudomonas is that we really only have six different drugs in the world that have any ability to kill this organism. And the organism is growingly becoming resistant to all six. So one of the reasons why we are on the hunt for new aminoglycosides is because they are looked upon as being the most powerful anti- pseudomonal drugs in the world. But resistance has developed so we are trying to develop new aminoglycosides that will be active against these multi-drug-resistant Pseudomonas.
FS: The biggest challenge we face in developing antibiotics is developing agents that work against gram-negative bacteria. Compared to gram-positive organisms, gram-negative organisms like Pseudomonas aeruginosa have two membranes which are impermeable for the majority of drugs, especially high molecular weight compounds above a molecular weight of 700-800 daltons. This molecular weight threshold is a particular challenge for the design of hybrid antibiotics where two or three antibacterial classes are merged into one molecule.
Is this where the idea of the gold nanoparticle come in, to act as a Trojan horse?
FS: Perhaps! The idea of presenting drugs on nano surfaces has created a lot of excitement in the drug research community. Initially, it was thought that drugs presented on nano surfaces could be more easily delivered into cells. Many promising results have been obtained over the years but most of these encouraging data have been obtained with eukaryotic cells. Very little success has been achieved with bacterial, especially gram-negative cells. This appears to be the big challenge.
This is scary stuff.
GZ: It is scary stuff because Pseudomonas is an organism that originally came from the soil. So it lives in the soil and what we found out is that the soil environment is the harshest environment to survive because if you go to soil, it is filled with nutrients. It is filled with bacteria, and fungi, and parasites. And all these organisms are fighting for the same nutrients. And how do you fight for nutrients? You develop weapons to fight and kill other organisms. And you develop defenses to prevent them from killing you. With antibiotics we are trying to do the same thing the organisms are doing to each other in the soil. Pseudomonas is winning the battle in the soil, and then it made its way into the hospitals with all this armour all ready to protect itself from antibiotics that it got in the soil. And now it’s in the hospitals and it has adapted to infect people, and it has all these defense weapons, so when we fire antibiotic missiles at it, it’s able to defend itself. It’s a huge issue.
So what needs to happen to tackle these gram-negative bacteria?
FS: [laughing] That’s a big question. You know, we have not developed a new class of antibiotics against gram-negatives in the last 30 or 40 years. Almost everything that has been recently developed is against gram-positive bacteria.
Big drug companies used to have genetic programs to identify new bacterial drug targets but unfortuneatly, this approach has not been successful. As it stands now we basically have no new targets. So chemists can make molecules, but if they don’t have new drugable targets where these things can bind to, it’s [pointless]. So what we are currently doing is going back to compounds that were developed in the 60s, which at the time were considered too toxic, and we are going back to these compounds to see if we can optimize their activity and reduce toxicity, but that’s a challenge as well.
The newest trend, coming back to penicillin, is asking ourselves, ‘Okay, why are these compounds no longer active?’ And we try to find ways to cope with that. To make them active again. So in the case of penicillin, you know it still has activity against certain strains of bacteria but others are resistant. And you know the mode of resistance – why the antibiotic no longer has activity. So you combine penicillin with something else that prevents the bacteria from destroying that drug. This is a more logical approach. You keep the drug but add something that prevents the resistance mechanism to occur or preventing it from inactivating the compound.
Do you foresee a future when antibiotics can be quickly tailor-made in hospitals to treat specific infections and specific patients?
GZ: I do. There are two different ways we go about business today. If someone comes into the emergency room at the Health Sciences Centre and they are very sick with a cough and a fever and chest pain, and they are coughing up thick secretions, we think that maybe this is pneumonia. And the state-of-the-art is you collect blood from the patient, just in case the organism got from the lungs into the blood, and you would collect sputum secretions from the lungs. And you would try and identify what the organism is. And the moment you send those samples into our laboratory, you put patients on antibiotics. And the antibiotics you put them on are there to cover all the potential possibilities that are causing the pneumonia. Two days later. Three days later. Four days later, if we’re lucky, our tests grow what the organism is. And now we say, ‘Okay, now we know what the causative agent is. Let’s change what we are doing and put on a very specific drug targeting for this organism.’
Four days later, patients are frequently dead, getting worse, getting better, or status quo. We need something where we can find out in half-an-hour or an hour what’s causing that pneumonia. And we and other groups are working on that – to collect blood, to collect sputum, collect urine, collect body tissue, collect brain tissue – to test it in a matter of 30 to 60 minute to find out what is the organism causing that infection. And then you can start a focused drug that will go right after at that organism. That is the way things have to go. We call this rapid diagnostics. If we develop these rapid diagnostics, then maybe we can treat the patient with a very targeting antibiotic that will kill only that pathogen and leave all the healthy bacteria that protect us alone.
So you receive superbug samples from across the country. What trends in the samples are you seeing?
GZ: The headquarters of the Canadian Antimicrobial Resistance Alliance, or CARA, is here in Winnipeg and as part of our CARA initiative we do national surveillance studies. We are looking across the country, whether it’s in the community or hospitals, for what kinds of infections are out there, what kind of organisms are causing them, what sort of superbugs are out there and where are they coming from, where are they going, and who are they infecting. And we’re seeing big trends.
One of the big trends we are seeing involves MRSA, Methicillin-resistant Staphylococcus aureus. This organism is a major, major cause of pneumonia, wound infections, urinary tract infections, bone infections, blood infections. This organism has done a huge turnabout in the last – I’m going to say, two to five years. It used to be that the patients who got MRSA were in the hospital. They were in the hospital for weeks, they were old, and frequently they had undergone surgery and had multiple medical problems. And they developed this MRSA in the hospital that caused an infection and they did very poorly. Very recently this organism has moved to the community and it now lives in the community and infects young, healthy individuals. We’re seeing this organism living in the community where large groups of people reside. For example, we’re seeing it in penitentiaries, in Aboriginal reserves, it lives in the university and causes infection among football players. The organism moves from person to person and it can infect football players who have cuts, turf burns, and bloody wounds from bashing into each other. This organism is growingly infecting the military, again large groups of individuals who have skin infections from performing exercises on the ground. So these organisms have moved from the hospital into the community, and they are causing very, very serious – sometime life-threatening – wound infections that don’t go away. So the organism has said, ‘You know what? I don’t have to just live in the hospital: if I make myself more able to cause infection, if I find new weapons, I can infect young, healthy individuals and start to spread through the community.’ That has terrified us. And the reason is, in the olden days, we were prepared for old people getting infected in the hospital. That’s kind of what happened. But now as they organisms are finding new weapons to infect young, healthy people. That’s terrifying.
Another organism worth mentioning is ESBLs. It’s a fancy term. E is extended. S is spectrum. B is beta and L is lactamase. These are organisms like E. coli, the most common cause of urinary infections in the hospital and the community. It is one of the top causes of respiratory infections in the hospital, a major cause of wound infections in the hospital and the number one cause of infections of the blood in the hospital. These organisms have developed resistance to all antibiotics. They have acquired DNA and resistance genes that make them resistant to multiple drug classes. We call them MDR – multi-drug resistant – some people call them XDR, extremely drug resistant strains. These E. coli are moving across the country, the United States and the western world and are becoming leading causes of infections – urinary, abdominal, blood – and they are resistant to virtually everything that we have. We never saw these organisms ten years ago. They have made their way into the hospital and they have made their way into the community. And they are very very difficult to stop. So we growingly have these organisms that in the olden days caused infections in the hospital setting only, but now they are finding ways to infect patients in the community; not just people living in nursing homes but people who live and reside in the community who are healthy. They are able to cause infections in those people. That’s pretty scary.
Are superbugs here forever then, always gaining virulence? Or do you think we can one day rob them of their “super” prefix.
GZ: I think they are here forever.
FS: This is more a question for a microbiologist but I don’t see why not. Bacteria will always develop ways of outsmarting drugs.
Research at the University of Manitoba is partially supported by funding from the Government of Canada Research Support Fund.